


(Clockwise) The Statue of Liberty (from Wikipedia), copper wires (from Demarco) and copper pipes (from Cleanipedia).
The first element of the blog: Copper!
The word copper comes from the latin word “Cuprum”, which roughly translates to “Cyprus metal”, as the island of Cyprus used to be a rich source of copper back when latin was big in ancient times.
Copper is such an ancient element in human history that we have no idea when it was first “discovered”, as copper ore can be found naturally in rock. However, the oldest evidence of copper smelting we have was found at Rudnik Mountain in Serbia, and dated to around 5,000BC. Therefore the Copper Age is thought to have been between 5,000 and 3,000BC. The age of copper was succeeded by the age of bronze, which was still technically a win for the metal of Cyprus.
This is because bronze, and brass for that matter, are alloys of copper. Alloys are the result of a smelting and mixing of two metals, in this case copper is combined with zinc to make brass, and tin to make bronze. The resultant alloys were stronger and more resistant to corrosion than pure copper; allowing them to be used in more robust applications like weapons and musical instruments.
That doesn’t mean copper can’t do well on its own though. Apart from silver, copper is the best conductor of heat and electricity of any metal of the periodic table. This is because of a lattice structure of copper, where positive copper ions are arranged in a regular structure, with electrons freely moving amongst those ions:
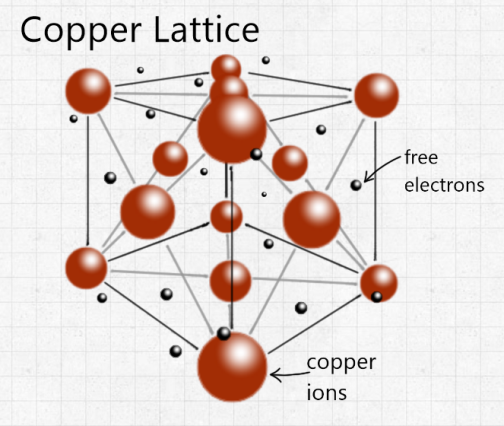
These free electrons are what allow electrical charge and heat energy to travel easily through the copper, making it a great conductor. This is why copper is often used in wires and cooking implements. Copper also doesn’t corrode easily, which combined with its relative cheapness means copper pipes are often used in plumbing. Copper is also biostatic, which means that bacteria and other microorganisms cannot grow on it. This is particularly useful in things we touch often, which is why many door handles are made of brass.
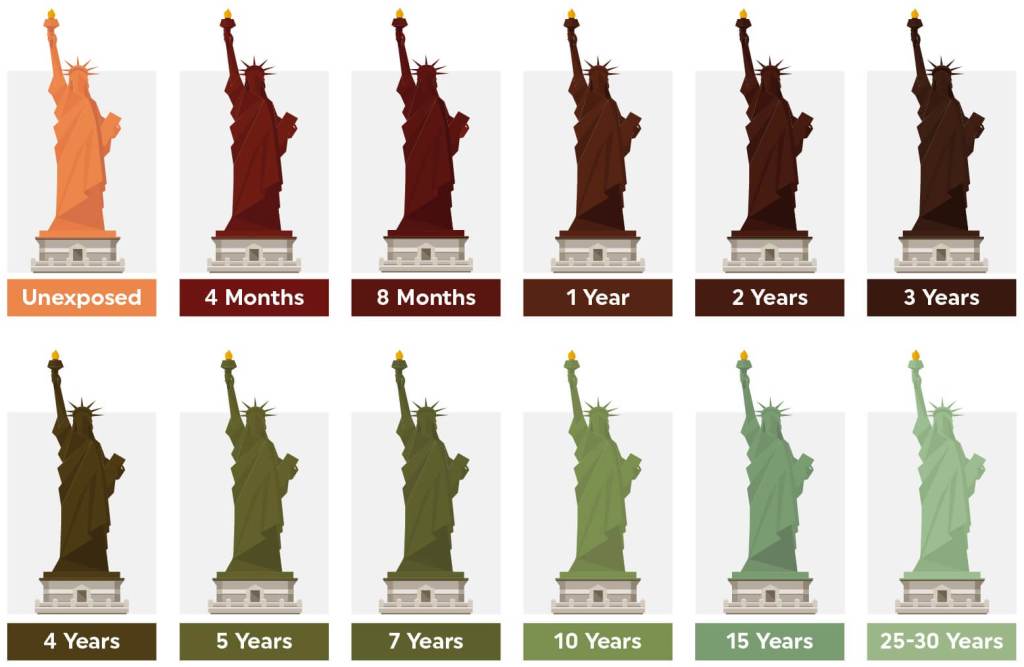
Copper is not entirely resistant to reacting with its surroundings. After continued exposure, copper with oxidise, reacting with acidic elements in the air to make a green substance called Verdigris. It is usually a mixture of copper carbonate or copper chloride, and explains why the Statue of Liberty (which is made of copper) is now a light blueish green. When it was made, the statue of liberty was the orangey-brown of copper!
So there it is, the metal of Cyprus now used for cooking, electronics and greeting immigrants seeking the American dream.

3 thoughts on “Day 1: Copper”