


(Clockwise from top left) Stacks of steel tubes (From Pikrepo), Iron as it appears on the periodic table, Lumps of pure iron (from Wikimedia Commons). Had to wade through a bunch of steam irons to find these ¬¬
Day three brings another classic metal element: iron!
The chemical symbol of iron, Fe, comes from the latin Ferrum. It is thought that the word “iron” itself comes from the Celtic word “isarnon”, meaning “powerful, holy” and “strong”.
Iron is another one of those elements where there isn’t really a “discoverer”, but did have an entire iron age that started roughly around 1200BC, although some civilisations did not start using iron until much later. Although iron was known about and some civilisations had started to smelt iron halfway through the bronze age, bronze was still preferred for quite some time. This was because copper and tin, the main components of the alloy bronze, were easier to extract from ore and to melt than iron, which needed a specialised furnace. Iron on its own is also not that much harder than bronze. However, the bronze age is thought to have “collapsed” around 1200BC due mainly to a lack of available tin, and therefore the use of iron started to accelerate.

One of the most famous ways to make iron stronger is through using it to make the alloy steel. Steel is typically iron mixed with the non-metal element carbon, although other elements may also be present (for example if you add the metal element chromium, you get the more rust resistant “stainless steel”). The earliest record of steel production was seen in around 1800BC, before even the iron age, in Anatolia (now the main peninsula of Turkey), although steel as a powerful industry wasn’t really established until the mid-19th century. This extra strength allows steel to be used in construction and cutlery.
Another great property of iron is that it can be magnetised. This means that it can not only be attracted to magnets, but also become a magnet! And to understand why, we must go to the atomic level. So every atom has electrons whizzing around it, which all have their own little magnetic field. Another thing about electrons is they often pair up if they’re at the same energy level, which cancels out their little magnetic fields as they point in opposite directions. However, unless they absolutely have to, electrons towards the outer “shell” of the atom will not necessarily pair up, and these electrons will not cancel out their magnetic fields, and in fact their fields can line up together to produce an overall larger magnetic field. If this happens at the atomic level, those atoms have quite a strong magnetic field! Iron happens to have unpaired electrons in its atoms.
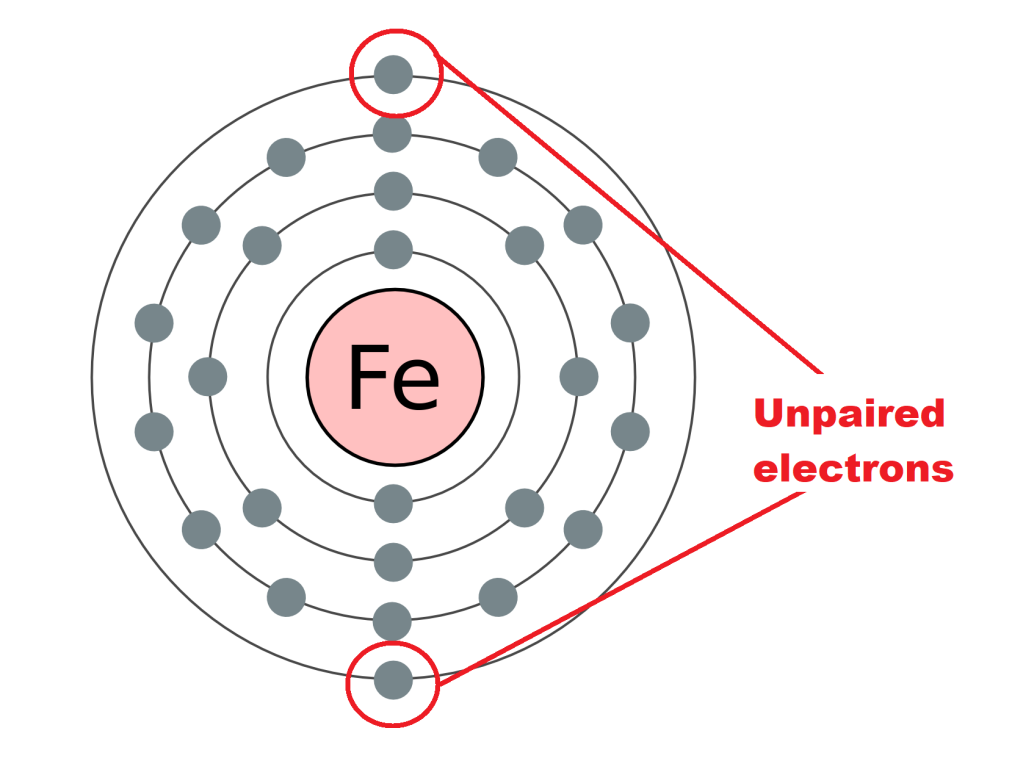
However, what is special about some elements, including iron, is that when all those magnetised atoms come together in a lattice structure to form the solid metal, those atoms line up so that all their magnetic fields point in the same direction, allowing the whole substance to be magnetic. This is not true, for example, with chromium, where the atoms alternate where they are pointing their magnetic field, cancelling out the overall magnetism. Whichever structure of atoms a substance uses is usually the one with the lowest energy.

So that explains how iron can be attracted to a magnet, but how can a lump of iron become magnetic itself? Well a lump of iron will consist of a bunch of “domains”, which are blocks of iron atoms arranged so that their magnetic fields point in the same direction. The problem is that each domain may have their magnetic field pointing in different directions cancelling each other out, meaning that the overall effect on the lump of iron is that it’s not magnetic. However, if you expose the lump to a strong magnetic field, eventually all the domains will line up their magnetic field to this big external one. Once all the domains are lined up, hey presto, you got a magnet! So iron is magnetic and can become a magnet because its electrons and lattice structure allow it to line up its tiny magnetic fields to create one massive one. Phew, hopefully that makes sense!
As a biochemist at heart, I couldn’t finish a this post without talking about how humans (and other animals) have been using iron since well before the iron age: in blood. In red blood cells, also known as erythrocytes (from greek: erythro=red, cyte=cell), there are protein called haemoglobin, which can bind oxygen and allows erythrocytes to carry this oxygen around the body from the lungs. Bound to each haemoglobin is a molecule called haem, which has in its centre-you guessed it- iron.

This iron has lost two electron from its outer shell, making it an iron ion (sounds a bit like Aaron Aaronson from Hot Fuzz…) with a positive charge. This helps the ion to bind the oxygen reversibly, and also gives the haemoglobin, erythrocyte and blood the classic deep red colour. Therefore once again iron’s unpaired outer electrons allow it to perform an excellent function!
So there we go, iron! The bloody magnetic construction queen of elements. I swear not all elements are named in specific ages…

Interesting- loved the explanation about how magnets work
LikeLiked by 1 person