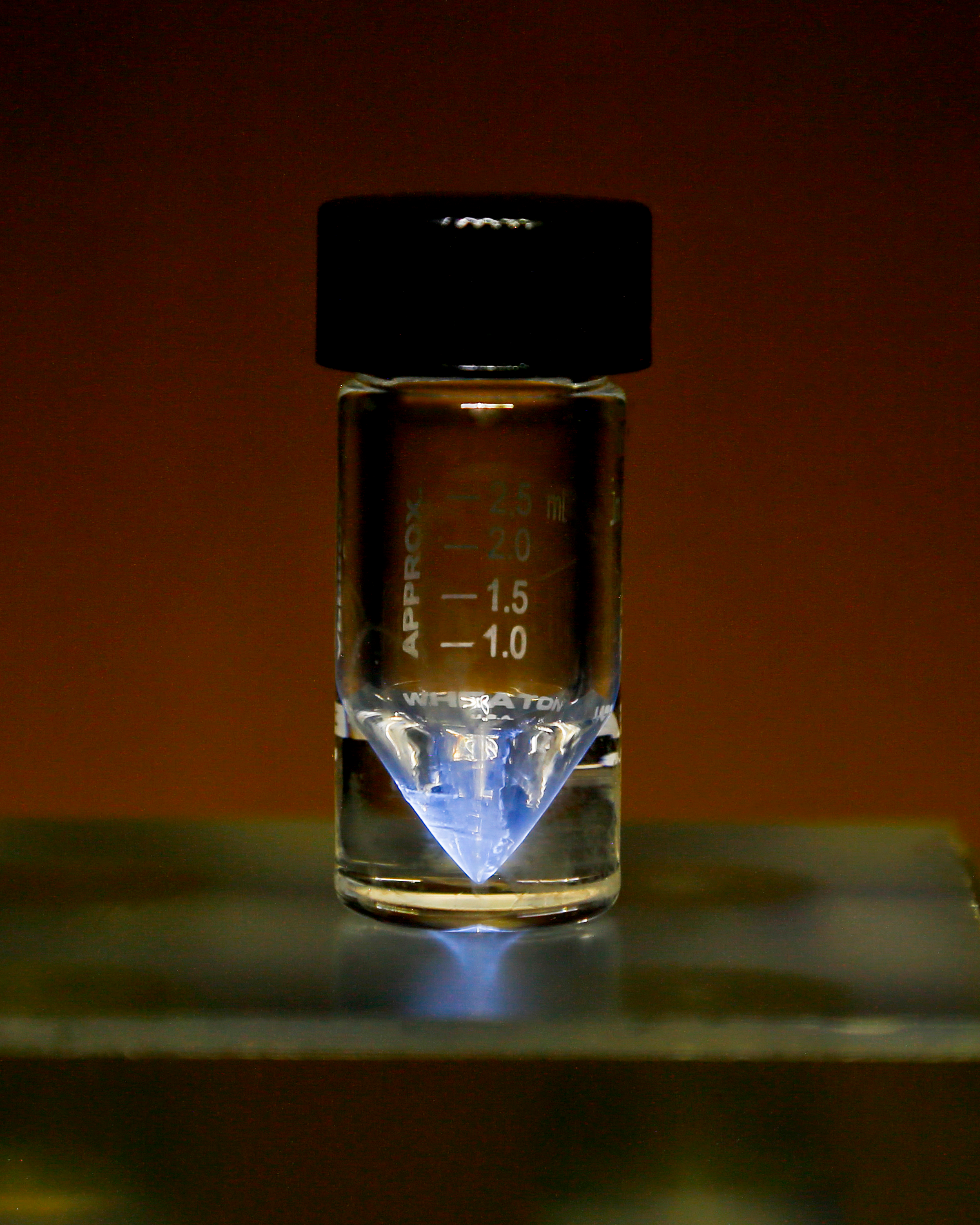

(Left) A sample of actinium (from Wikimedia Commons).
Rounding off the first week of the blog we have another radioactive friend: actinium!
So what can we say about actinium? Well to start off with, it glows! Exactly what people want to hear about radioactive substances, right? It’s because the radiation coming off actinium is so strong that it ionises the air. This means that energy from the radiation is being absorbed by nitrogen and oxygen molecules, which enters them into what’s called an excited state (who doesn’t have extra energy when excited?). This energy is then usually released from the molecules in the form of light energy, with nitrogen emitting a purple light and oxygen emitting blue. So when sci-fi and cartoons have radioactive elements glowing, they’re not that far off!
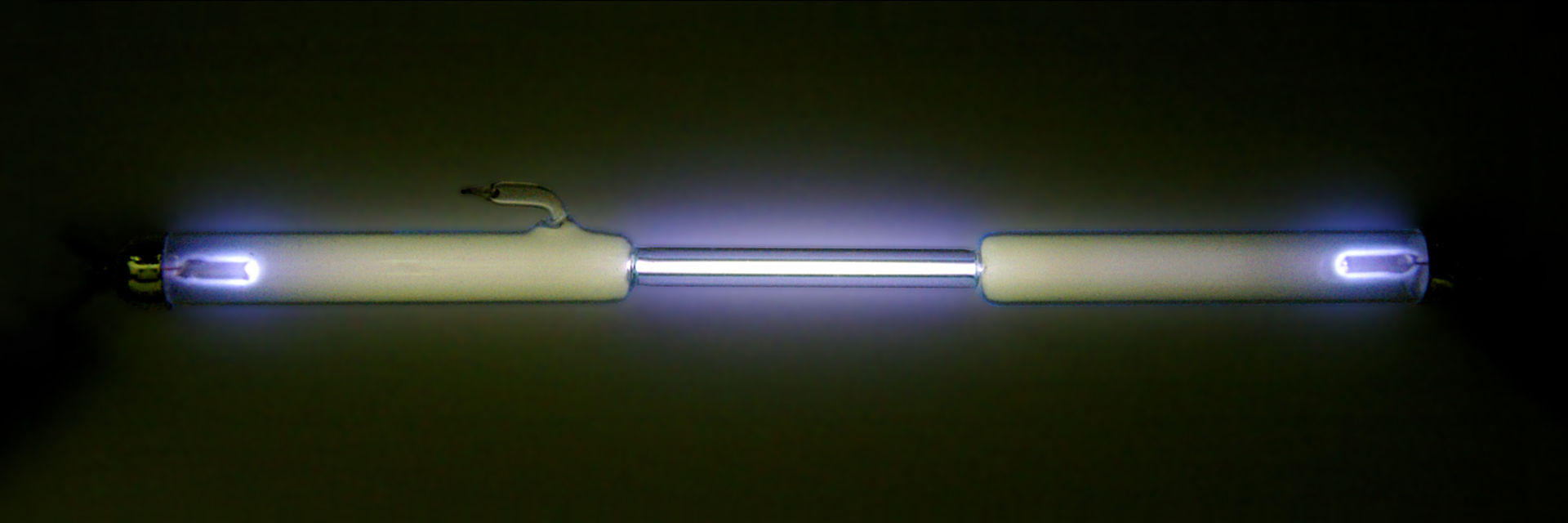
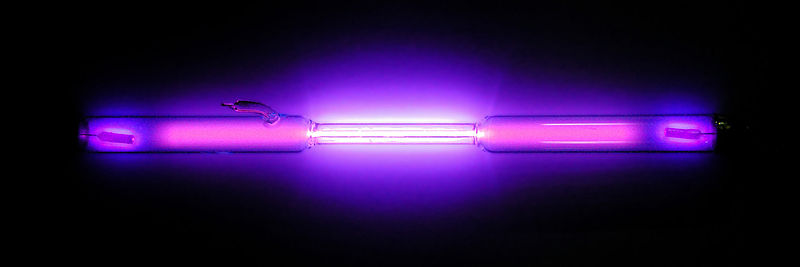
Oxygen (above) and nitrogen (below) glowing when ionised. From Wikimedia Commons.
Actinium is also an element that was discovered twice. The first discoverer was André-Louis Debierne, who in 1899 managed to find it in a substance called pitchblende, a predominantly uranium-rich substance that led to a lot of radioactive element discoveries. In fact, the pitchblende Debierne was extracting actinium from had been previously used by the Curies to extract radium. Debierne named the new element actinium, from the ancient Greek “aktinos” meaning ray.
The second discovery comes in 1902 when Friedrich Oskar Giesel managed to isolate actinium and claimed he had founded a new element, which he named emanium. However when the rate of decay of the two elements were compared, it was found that they were in fact the same element, which kept the name actinium in a “finders keepers” kind of way. However, there was some controversy about whether the substance Debierne submitted for analysis was the same as the substance he worked in in 1899. Giesel was also the one to correctly identify the atomic number of actinium and provided the first proper way to prepare the pure element, meaning that Giesel’s contribution to actinium’s discovery should not be swept under the historical rug.
So that’s actinium! But I hear you ask “what are its uses?” Well unfortunately it is such a rare and strongly radioactive substance (did you read the bit about it GLOWING?!) that there isn’t much that we can do with actinium, past some people looking into using it as either a source of radiation for experiments or to target cancer cells and kill them in radiotherapy (see the post on astatine). But sometimes science is about figuring out stuff that may seem pointless now, yet in the future may have a use we didn’t consider or have the capacity to understand. Actinium may gain an essential use to us… eventually…

One thought on “Day 7: Actinium”