

Clockwise from top left: Some lumps of zirconium (from Wikimedia Commons), Zirconium as it appears in the periodic table, cubic zirconia, an alternative to diamonds! (from Needpix)
For day 11, here’s the final element you’ll find in the dictionary: zirconium! An element whose famous mineral has been known about for a long time (and is mentioned in the bible), but was not known to be an element until 1789, with help from a reoccurring name in elemental discoveries.
The mineral in question is zircon, and early references to this zirconium-containing compound are what eventually gave it its name. Zircon is a crystal of zirconium silicate (a compound containing zirconium, silicon and oxygen) and has a distinct yellowy hue to it. In fact, the word zircon is thought to have come from the Persian word for gold-like: “zargun”. It is usually added to things like ceramics to make them opaque, which is known as an opacifier. But the discovery that zircon contained a new element didn’t come until 1789, when our good friend Martin Klaproth analysed a form of zircon that is often used in gemstones called jargoon. He named it Zirkonerde, which roughly translates to zirconia, but it was eventually changed.

So zirconium has a couple of interesting properties that allow for various uses. It is incredibly heat resistant, with a higher melting temperature than even platinum and titanium. This means as well as the aforementioned ceramics (including ceramic knives!), zirconium is used in metallurgic furnaces, spacecraft and jet engines. In medicine, zirconium has use in prosthetic devices due to it being biocompatible, but also has a strange property in that it can bind to the chemical urea, a waste compound that is excreted from the body in, you guessed it, urine. This lead to zirconium being used in dialysis machines between 1973 and the 1990s as a “urea-absorber”, filtering out the substance in patients with chronic kidney disease, as a build up of urea in the body will get toxic. So zirconium will protect your astronauts and absorb toxins from your pee, what a nice element!



Clockwise from top left: A big space shuttle (from Pikrepo), a ceramic knife (from Wikimedia Commons), and some metallurgy (from Pikrepo). Decided the kidney dialysis function can just be for your imaginations.
The final thing about zirconium I wanted to bring up today is the most aesthetically pleasing: cubic zirconia. Many chemical compounds have a crystalline structure, the classic example being salt (sodium chloride), where the atoms in the compound have such a regular, repetitive structure to it that it readily forms a crystal. Well cubic zirconia is such a compound, as it is made of zirconium and two oxygen (zirconium dioxide). This crystalline structure comes together to form a very hard, durable and colourless gemstone that looks an awful lot like a diamond. You can even colour cubic zirconia by adding other substances whilst its being made, meaning that there are a rainbow of wonderful gemstones often used as a cheaper substitute in jewelry.
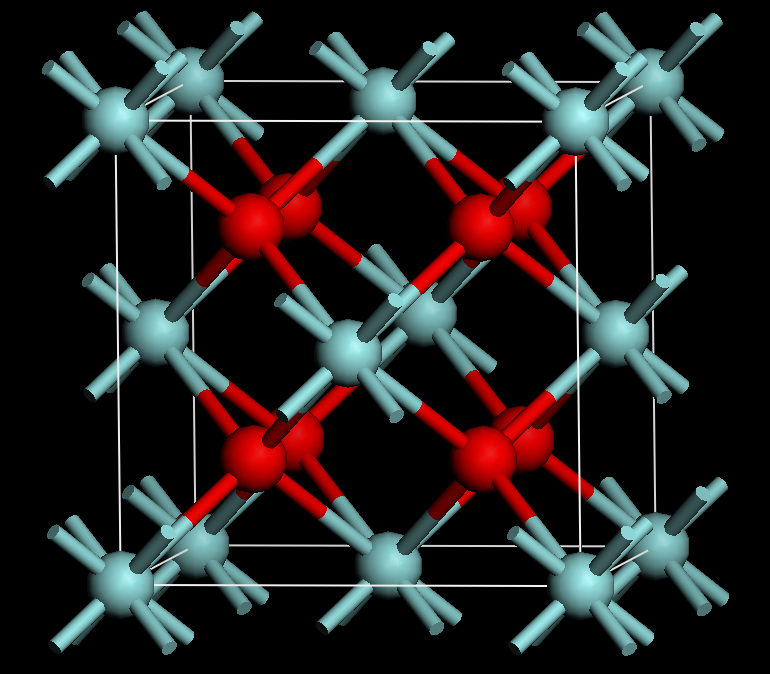

Left: the crystal structure of cubic zirconia. The red spheres are zirconium, the blue oxygen, with the rods being bonds between them. For every zirconium, there are two oxygen. Right: the colouring of cubic zirconia can make some beautiful gemstones (both taken from Wikimedia commons).
Zirconium: the heat-resistant, urea-binding gemstone of the elements! And an excellent word in scrabble.
