


Clockwise from left: Rubidium in use for purple fireworks (from Needpix), Rubidium as it appears in the periodic table, a vial of rubidium (from Wikimedia Commons).
Ahh rubidium. Any fans of caesium may see some parallels with rubidium, as the former is found directly below the latter in the periodic table. Elements that fall in the same column (or group) of the table will often have similar properties as they tend to have the same number of electrons in their outer electron shell (for group 1, that happens to be 1). The single electron in the outer shell is very easy to pass on to another atom (i.e. an atom that just has one electron missing from its outer shell), and therefore the elements in group 1 often do this to become positively charged ions.
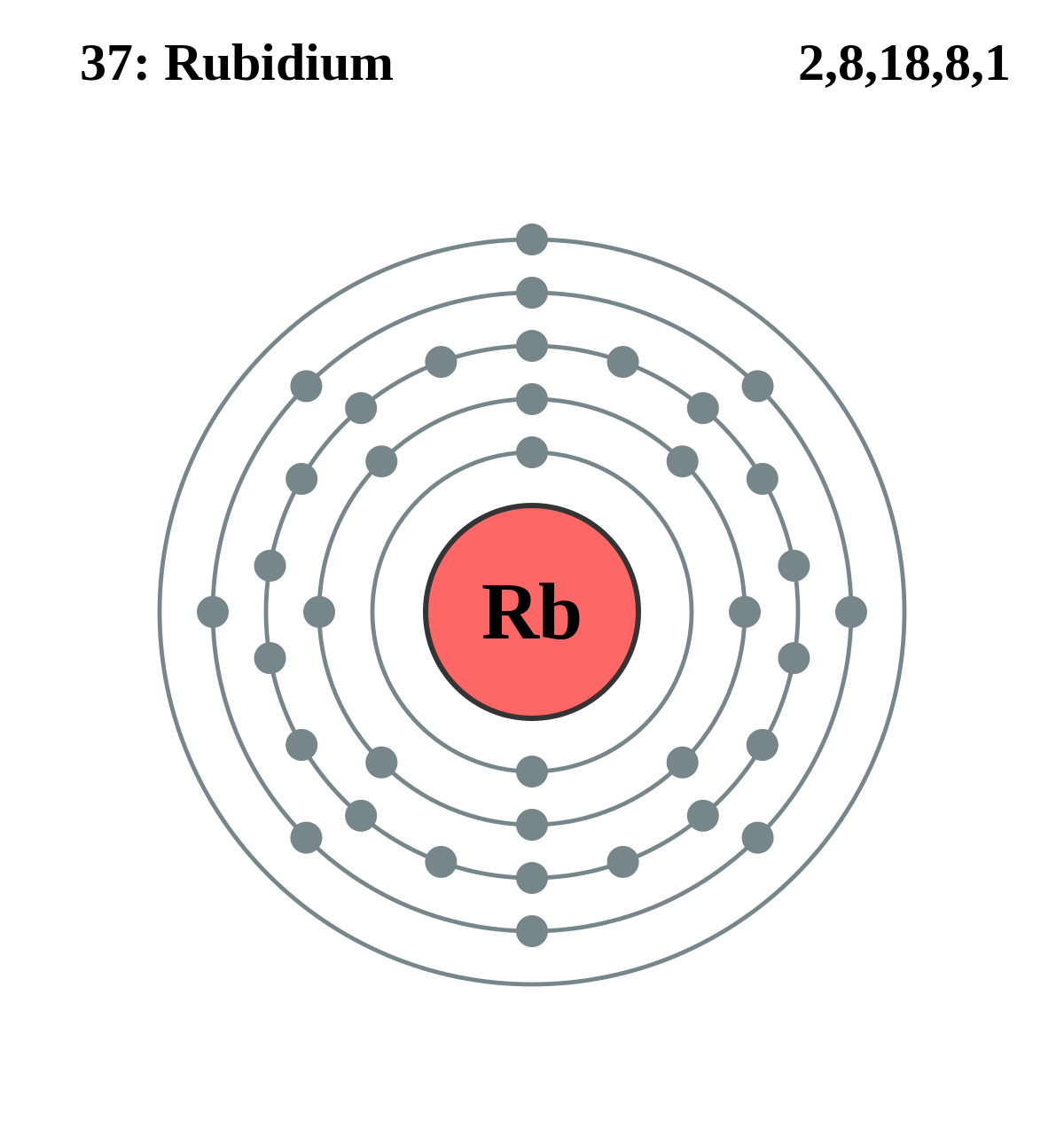
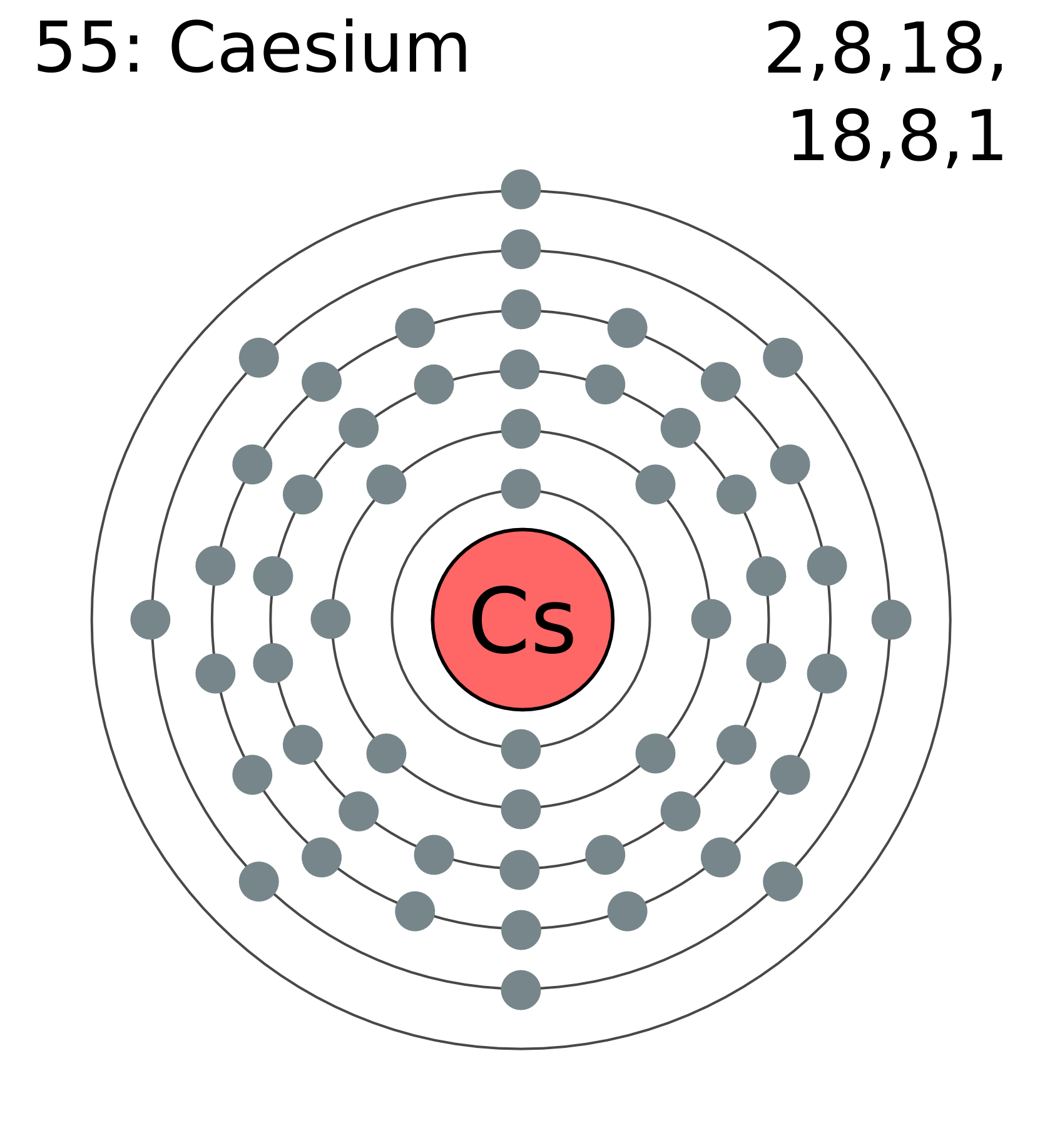
Rubidium (left) and caesium (right) have the same single electron in their outermost shell, giving them similar properties. (From Wikimedia Commons.)
Even the method of discovery and etymology of rubidium smacks of caesium! It was discovered by Robert Bunsen and Gustab Kirchhoff in 1861, just one year after they discovered caesium, using exactly the same method: flame spectroscopy (see my post on caesium for how that worked). This time when they burned the substance the spectrometer showed a red colour, leading to them naming the new element rubidium, from the Latin “rubidus”, meaning “deep red”.
So if rubidium has similar properties and discovery to caesium, does it have the same uses? The answer to this is the answer you’ll almost always get from a scientist: mostly. Like caesium, rubidium is used as a standard in atomic clocks, which are often used in processes like GPS where accurate timekeeping ensures accurate location data. However rubidium is not as dense as caesium, and so is not used in density centrifugation or drilling so much.
Rubidium instead is used to produce a purple colour in fireworks, as when burned rubidium compounds give a nice reddish-violet flame. Rubidium’s similarity to another group 1 element, potassium, allows for rubidium atoms to accumulate in the human body where potassium usually would. This can be very useful in imaging various parts of the body in medicine, as radioactive forms of rubidium can accumulate in brain tumours where it can be detected using positron emission tomography (PET) scans and its pumping around the vascular system can indicate the functioning of the heart.
Rubidium can also used in specialised photocells- devices that when hit by light produce an electric current. As well as the obvious use in solar panels, this can also be useful for motion detectors, whereby a beam of light is fired across, for example, a corridor into a photocell. If that beam is interrupted in any way, then the electric current stops for a moment and the alarm can be activated. So those laser grids in spy movies? Might be using rubidium!

So that’s rubidium! A reddish purple, medicinal spycatcher of an element!
