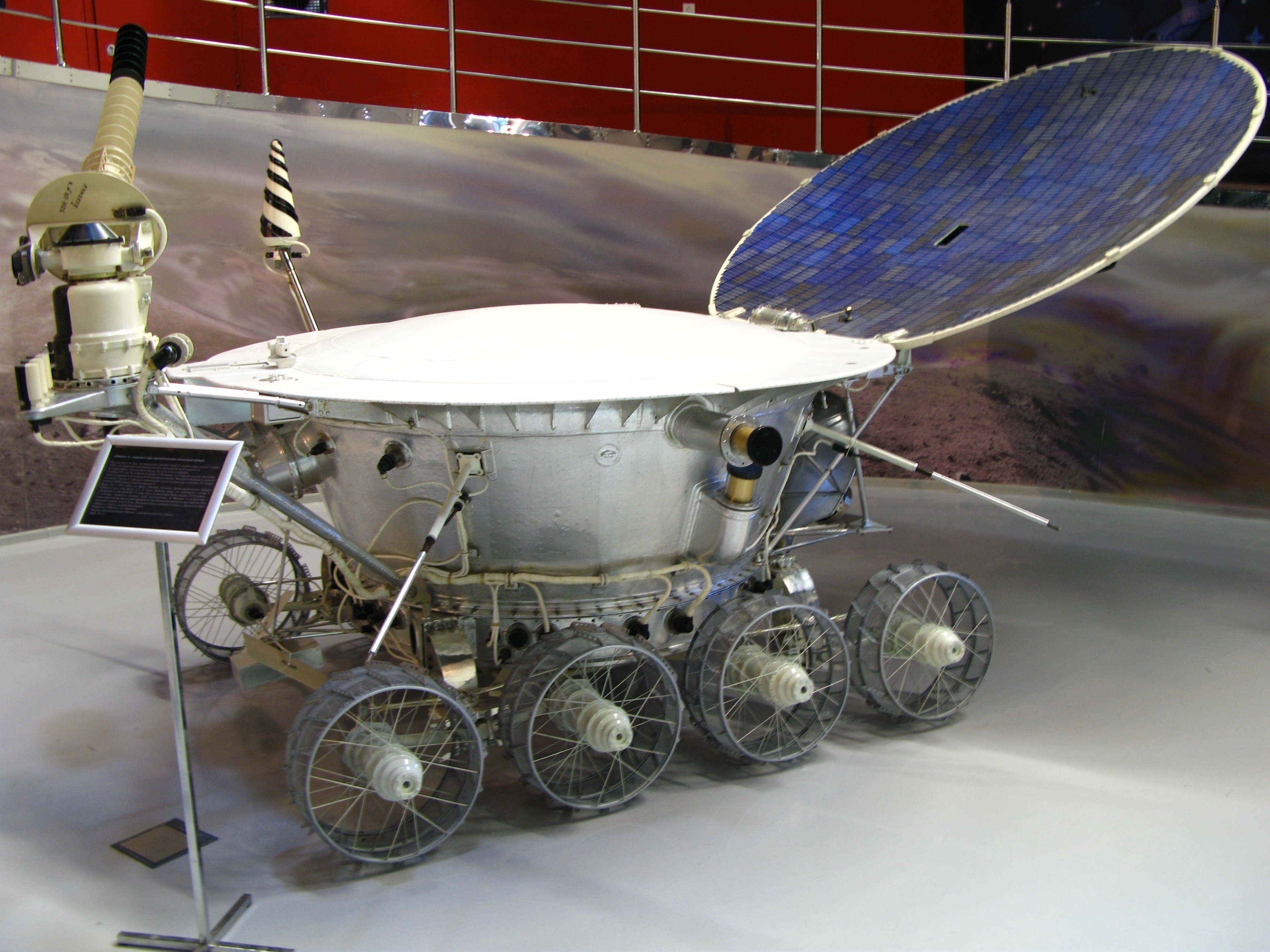

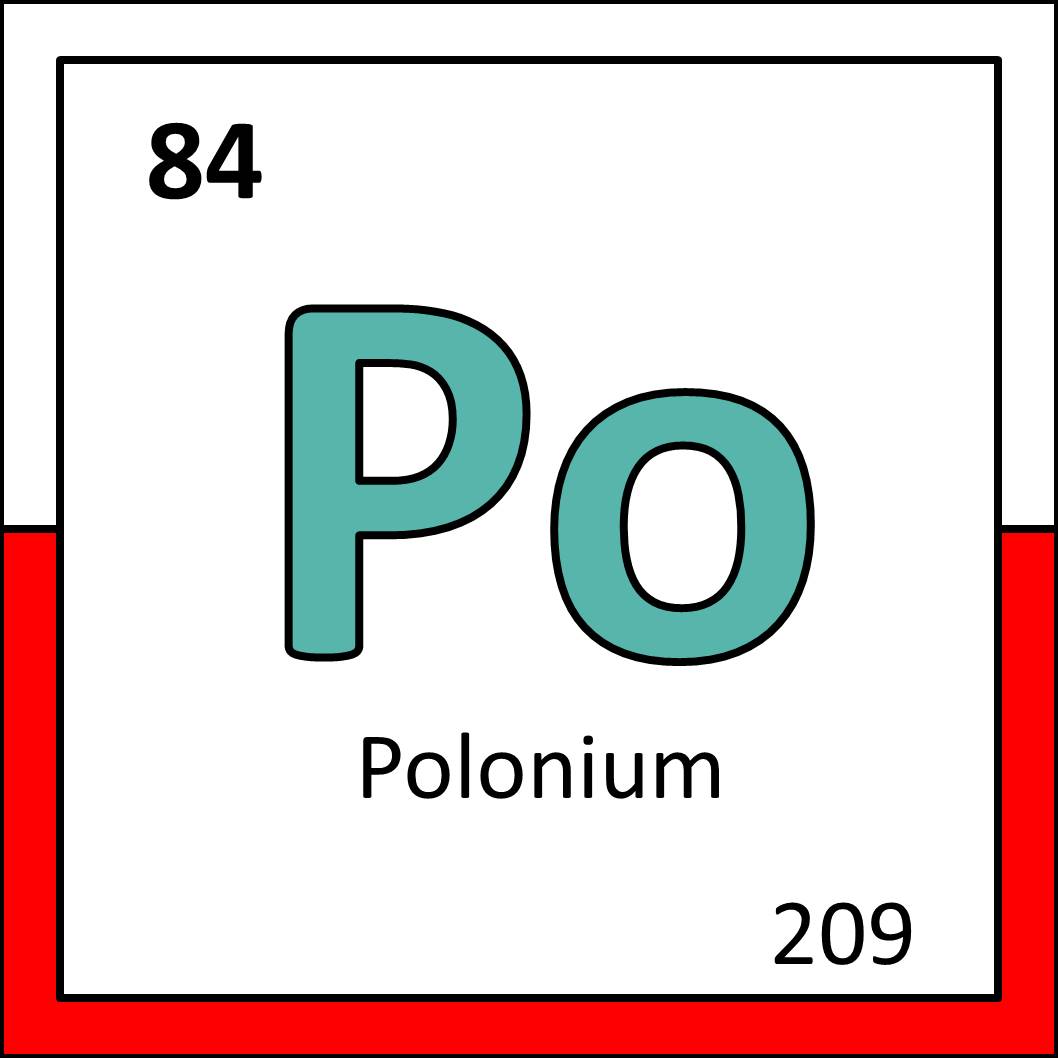
Clockwise from left: Lunokhod 1, a soviet moonwalker and user of polonium (from Wikimedia Commons), a tiny disc of steel with a film of polonium on it (from Wikimedia Commons), polonium as it appears in the periodic table.
We’ve returned to radioactivity and pitchblende with polonium! Unlike the weak radioactive thorium from two days ago, polonium is highly radioactive, with the most stable form having a half-life of 138 days!
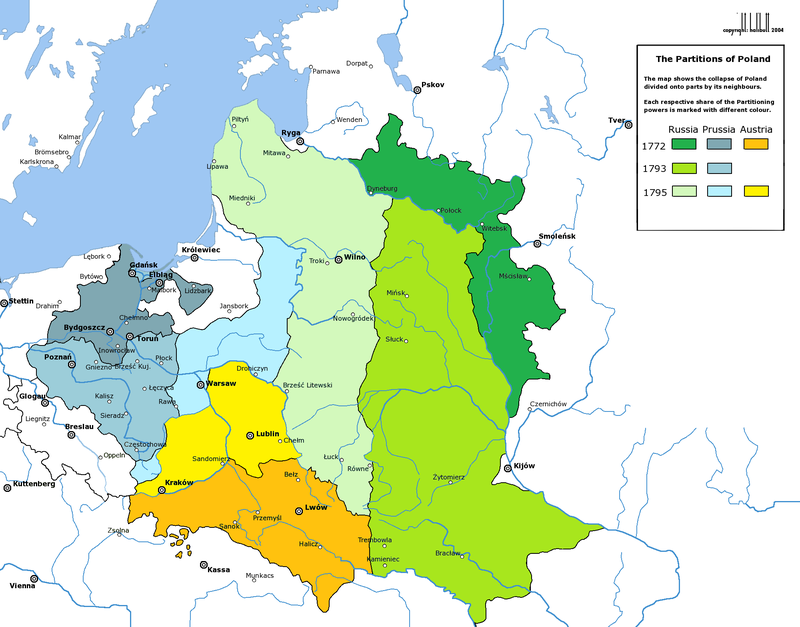
Polonium was discovered by the mother of radioactivity, Marie Curie, and her husband Pierre. The Curie’s noticed that when they removed the uranium and thorium from pitchblende, the resultant mixture was still highly radioactive- moreso than either of the elements they just extracted! This led them to extracting radium and polonium (originally called radium-F) from the pitchblende in 1898. Curie named the element polonium after her homeland of Poland, a country that at the time was under partition from Russia, Germany and Austro-Hungary, in the hope that it would gain its independence. Polonium was an attempt to raise awareness of the issue!
Polonium’s high radioactivity led to a lot of applications throughout the 20th century that are largely no longer due to health risks and the short half life reducing longevity of products. Polonium was used in the triggering of nuclear bombs during the Manhatten Project in the 1940s as it strongly gives off neutrons whilst decaying. The Soviet Union also used polonium for measuring how thick industrial coatings were by allowing the element to release alpha particles through them and seeing how many got blocked. The Soviets also used the fact that the radiation coming off polonium was so intense that it heats itself up to over 500°C to keep the components of their moon rovers Lunokhod 1 and 2 warm on the cold surface.
A slightly stranger use of polonium came in the 1940s and 50s from a tire company in the US called Firestone. They sold polonium spark plugs, that they claimed would improve the firing of the engine through ionising the fuel in the engine’s cylinder. Unfortunately the product was short lived, literally, due to the half life of polonium. Finally, polonium was used in anti-static brushes, as the radiation from polonium could ionise the air near the surface of an object, which would then neutralise any charges of said object. Again, it was the half life of polonium, rather than its hazards, which caused these brushes to eventually be replaced.

So that’s polonium: a politically aware, dangerous but also warm element!

One thought on “Day 34: Polonium”