

(From left to right) A good old lump of arsenic (from Wikimedia Commons), arsenic as it appears in the periodic table, proponent of arsenic cosmetics Queen Elizabeth I (from Flickr).
One many people have heard of before, arsenic has an infamous air around it for being the most famous elemental poison (cyanide not being a pure element, but a carbon and nitrogen bonded together, CN). It has been around since the bronze age as a part of the alloy bronze itself, although it is unclear whether its inclusion was deliberate or due to arsenic present in the copper ore. Either way it does allow the bronze to be stronger and more easily worked.
There’s subsequent evidence of arsenic use in Ancient Egypt, Rome, China and Greece. In these ancient civilisations arsenic was mainly known through its oxides and the sulfide minerals realgar and orpiment. Realgar is a beautifully bright red mineral that was known as a poison and was used for weedkiller, insecticide and rat poison, as well as for white fireworks. Orpiment is an opaque yellow mineral that can form from realgar decaying, which was also a known poison and used for poison arrows. However it was also used for medicine in China as well as alchemy in both China and Europe due to the fact it has a similar colour to gold. It is in fact this colour that gives arsenic its name, as it is beleived to have originated from the Arabic al-zarnīḵ (الزرنيخ) roughly meaning “yellow orpiment”. The first isolation of arsenic was thought to be by Persian Jabir ibn Hayyan, with its identification as an element being credited to German saint Albertus Magnus.


The minerals Realgar (left) and orpiment (right). From Wikimedia Commons.
A strange theme around arsenic’s history does seem to be its simultaneous use as a medicine and a poison. Arsenic poisoning does not have very specific symptoms, with some sources comparing them to those of cholera, which made it a favourite for murder. This combined with how potent a poison arsenic is has led to it being considered a favourite for royals looking to accelerate their inheritance of power, leading to arsenic being called the “king of poisons” and “inheritance powder”. The way arsenic kills is by disrupting an enzyme called pyruvate dehydrogenase, which shuts off the way cells generate energy, leading to their death.
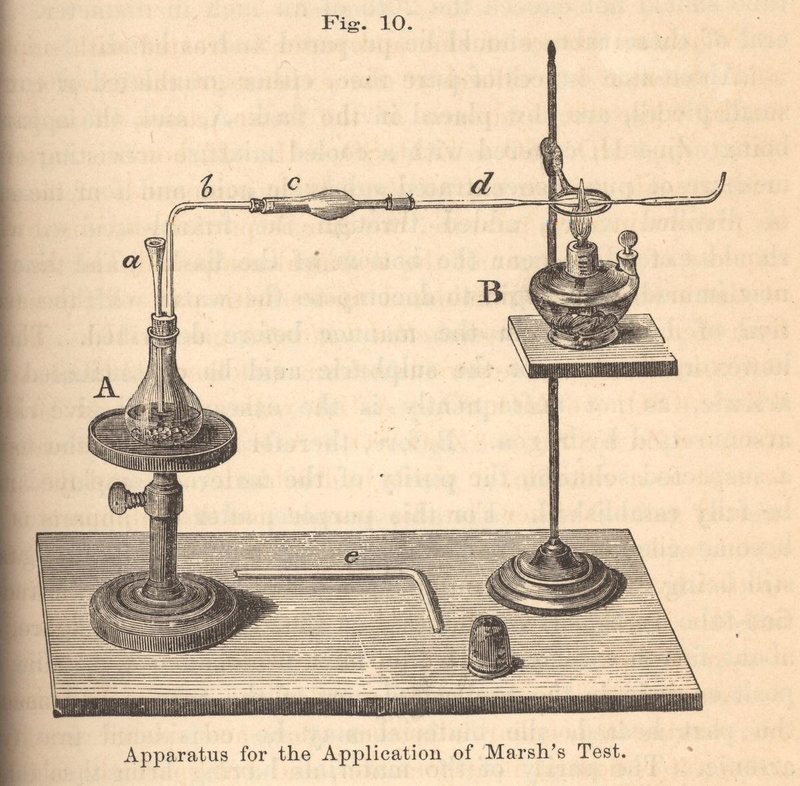
Unfortunately for arsenic and murderers, in 1836 James Marsh published a highly sensitive test for arsenic in body fluids or tissues. It involved reacting the sample with acid and zinc, producing arsine gas if arsenic was present. This gas was then ignited, which would generate arsenic from the arsine gas, which would cause a cold bowl near the flame to gain a silvery-black stain. This Marsh test was not only the end of arsenic-murderers getting away with the poisoning, but also the first example of forensic toxicology recorded!
The medical uses of arsenic also span from ancient China to the 20th century. Arsphenamine, also known as salvarsan, was used between 1910 and 1942 as a treatment for syphilis and trypanosomiasis, as it was fatal to the pathogens causing them. Although it was largely considered the first modern antimicrobial, it was replaced by penicillin. Dr Fowler’s Solution (potassium arsenate solution) was a famous Victorian “cure-all” tonic (never trust something that claims this) developed in 1786 and used right up until 1905. Charles Dickens was apparently a fan.
Another worrying use of arsenic was in cosmetics. In the Elizabethan times upper class women mixed chalk, vinegar and arsenic (known as white arsenic) and then rubbed it on their skin, in an attempt to make the skin paler and prevent wrinkles (again not advised). In the US in the 19th century drug this was taken further when women were encouraged to eat some white arsenic to remove skin blemishes. All of the time the use of arsenic as a poison was well known!

A final old use for arsenic was in pigments. Paris Green, also known as copper acetate triarsenite, was a toxic green pigment popular amongst such painters as Turner, Monet, Renoir and Van Gogh. As the popularity of arsenic started fading out, Paris Green started gaining use as an insecticide, until it was replaced by the equally problematic DDT. Another pigment, Scheele’s Green, was even used to colour sweets!

Nowadays arsenic is mostly used in an alloy with lead, as it will strengthen the metal and decrease corrosion. Therefore there may still be small amounts of arsenic in car batteries and some circuits in solid state hard drives (gallium arsenide semiconductors), but its not all that common.

2 thoughts on “Day 48: Arsenic”