

Clockwise from left: Some lumps of sodium sitting in oil to prevent the surface reacting with oxygen (from Wikimedia Commons), the inevitable salt picture that comes with sodium (from Pxfuel), sodium as it appears in the periodic table.
An element that is quite well known to a lot of people, sodium is almost synonymous with salt. But I assure you, sodium can be much more than that!
Sodium in its various forms has been known about for a long time, and these historical applications lend sodium and its Latin term natrium their names. Soda (sodium carbonate) has been used for millennia for various purposes, one of which is to alleviate headaches. In fact, it has been speculated that the word soda comes from the Arabic “suda”, meaning headache, although the words suwwād (saltwort) and suwaydāʾ (seepweeds) have been put forwards as the origin as they are plants that would grow in very salty areas. Natrium comes from the Ancient Egyptian natron (a mixture of sodium carbonate and sodium bicarbonate), which was used in preserving organs and mummies, as it would dry out the bodies. This all came together when Humphrey Davy (he’s back!) managed to isolate sodium, and whilst he proposed “sodium” as a name, his German rival Ludwig Gilbert suggested “natronium”. Just like in the case for potassium, the compromise was that sodium was the word, and natronium was used for the chemical symbol (Na).

Sodium as a pure metal is highly reactive: it needs to be kept in inert gas or oil to prevent it reacting with the air around it. Taking the shiny metal out of this environment results in the sodium slowly turning a dull grey as the outside of it reacts with the oxygen in the air to form sodium oxide. If you want to see something fun, watch a video of pure sodium dropped in water, where it reacts so strongly that it whizzes around on the surface of the liquid (it is lighter than water), producing sodium hydroxide and hydrogen gas (the latter of which propels it around). Sometimes the heat given off from the reaction is enough to ignite the hydrogen, setting the sodium ball on fire.
Most of the uses of sodium come through one of its salts. Sodium chloride is used as a preserving agent (and flavour of course!) and for de-icing surfaces. The salt dissolves in the small amount of water on the ice surface, which lowers the melting temperature of the water and subsequently melts the ice underneath, which dissolves more salt and so on. Sodium carbonate or soda ash can be used to clean clothes, to make silica easier to melt into glass, and for water softening, as the sodium replaces heavier metals like calcium and magnesium. Sodium carbonate can dissolve in water, producing positive sodium and negative carbonate ions. The positive magnesium and calcium ions in the water (which makes it hard) then bond with the carbonate ions, which are insoluble and can be filtered out of the water. Sodium bicarbonate (or more commonly bicarbonate of soda) is often used in cooking as a leavening agent (its foaming puts air in mixtures like dough), and was the main component of those black snake fireworks, which uses the carbon dioxide given off when the sodium bicarbonate breaks down when lit to puff out the carbon produced from the decomposing sucrose (sugar) in the starting material. The overall effect is a black snake starts to appear seemingly out of thin air!

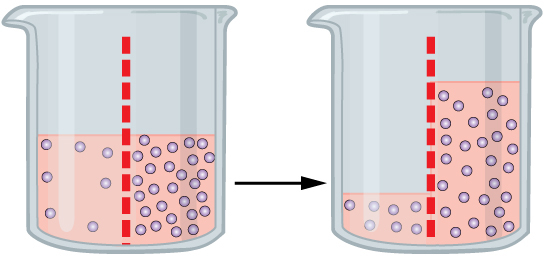
I thought I’d finish off with some classic biology, and sodium is a very important element to have in your body. If you remember from my posts on potassium and calcium, charged ions are used by the body for many purposes, such as spreading charges and signalling between and within cells. Sodium is the most common positively charged ion in the body for these, but also serves another role: regulating blood volume and pressure. I’ve briefly discussed before the idea of osmosis- that when two compartments of water are separated by a partially permeable membrane (they let some things across it), the net movement of water will be into the compartment with more dissolved chemicals within it (a higher concentrated solution). Effectively what this means is that the body can shift sodium ions into and out of the blood to control how much water will shift from the cells into the blood and vice versa. Detecting the relative ratio of sodium ions to water in the blood can also allow your body to respond to restore balance (a process called homeostasis). If the concentration of sodium in the blood is high, your body will signal thirst. If the concentration of sodium in your blood is low, your body will signal “pee”. So sodium, strangely enough, will help you stay hydrated!
So that’s sodium! One final fact to leave you with: salt used to be given in thin discs to Roman soldiers when they got paid, which were called salarium. And this is where we get the word “salary”.

3 thoughts on “Day 52: Sodium”