
Clockwise from left: technetium as it appears in the periodic table, a red giant star which produces technetium through nuclear reactions (from Wikimedia Commons), a nice shiny lump of technetium (from The Guardian).
We’re sticking with our radioactive elements with technetium, which is actually the lightest (lowest atomic number) element where all its isotopes are radioactive!
It was also the first element to be artificially made, but before that there were many attempts to discover element 43. When Mendeleev created the early periodic tables, there was a gap between molybdenum and ruthenium, and from the 19th century into the early 20th many people claimed to have filled that gap. Names like polinium, ilmenium, pelopium, davyum, lucium, nipponium (which we talked about in the rhenium post) and masurium were put forwards, but each time the discovered substance was either something other than element 43 or the results could not be repeated.
Italy is the country to take credit for the official discovery in 1937 with Carlo Perrier and Emilio Segrè being the victors. After visiting the cyclotron at the Lawrence Berkeley National Laboratory in the United States, Segrè asked for a piece of the molybdenum used in the machine from Ernest Lawrence (inventor of the cyclotron). Segrè and Perrier managed to isolate some element 43 from this molybdenum foil, that had been made after bombardment with radiation. After initially discussing panormium as a name (using the Latin Panormus meaning Palerno, their home city), technetium was eventually settled on using the Greek τεχνητός, which means “artificial”.

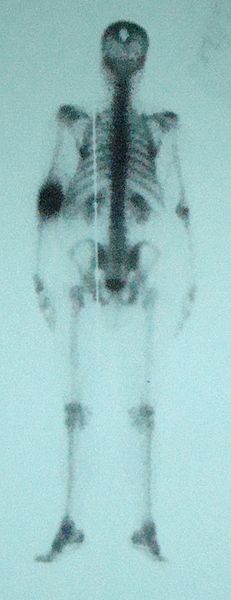
Technetium’s radioactivity means its uses are limited, but there are still some! Technetium can be bound to a pharmaceutical chemical which can result in its transport to specific places in the body, which combined with detectors picking up on the radiation let off from the element means that parts of the body can be imaged for diagnosis. This process, called scintigraphy, can allow for the brain, heart, thyroid, lungs, liver, gallbladder, kidneys, skeleton, circulatory system to be analysed and can even be used to detect tumours. The form of technetium used (technetium-99m, with the m standing for metastable, which means it is not the most stable form but still pretty stable) is considered one of the most common medical radioisotope in use.
Technetium also has a mysterious ability to keep steel from rusting when a solution of the element surrounds the metal. This has led to some suggestions for technetium salts to be used in applications where steel needs to be in contact with water (e.g. boilers), but the radioactivity of technetium brings up concerns. Similarly, technetium can act as a very good catalyst for removing hydrogen from isopropyl alcohol to make acetone, but the radioactivity means that extra protection and extraction would be needed.
The final thing about technetium is that it taught us that stars are capable of creating quite heavy elements through nuclear reactions. In the 1950s spectroscopy being performed on very old red stars gave very specific coloured bands of light that would suggest technetium was abundant. Although the existence of smaller elements was documented in stars, this provided excellent evidence to show that heavier elements could be created in these astral bodies through nuclear reactions within!
So that’s technetium- a rust-resistant, artificial(ish) and stellar element!
