
Clockwise from left: a huge vat of liquid oxygen at the Cape Canaveral rocket launch site (from Wikimedia Commons); a diagram of the respiratory tract and lungs, whereby oxygen can get into our bloodstreams (from Pixabay); oxygen as it appears on the periodic table.
It’s the big 100! And what better way to celebrate the 100th element blog post than with the other big element of life (bar carbon)- oxygen. And oxygen is an interesting one, because as much as it provides us with life, in the wrong form it can be incredibly dangerous.
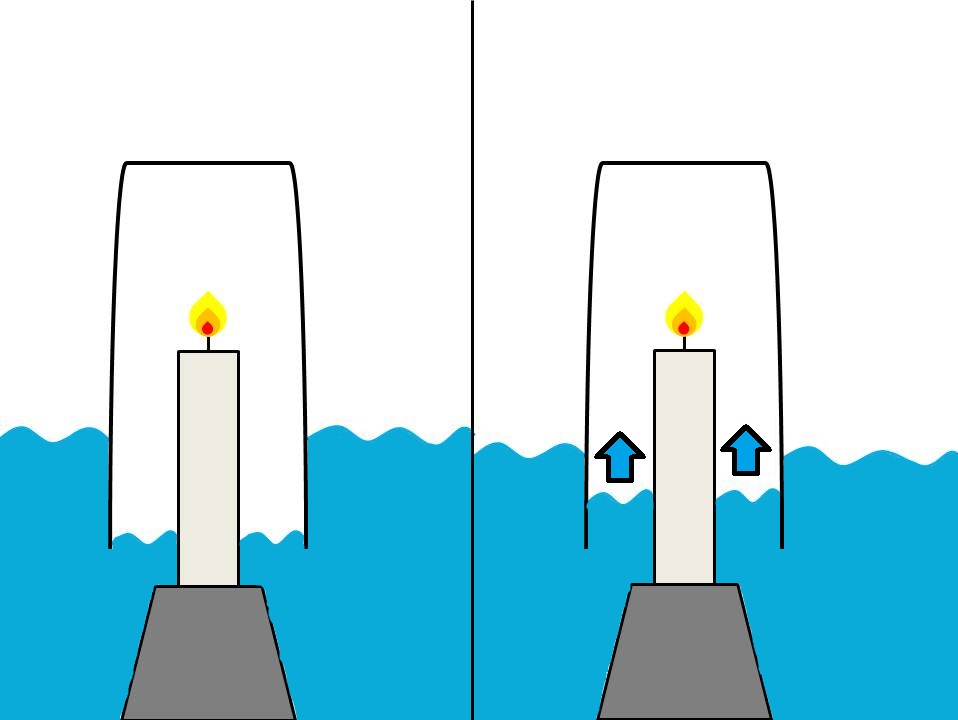
Oxygen has gone by many names throughout history, as thinkers through time are trying to figure out this invisible substance that we breath to survive. It was in the 2nd century BC where the first connection that we know of was made between combustion and the consumption of air. Philo of Byzantium, Greek engineer found that when he put an upside down container over a lit candle and surrounded the container with water that some water started slowly travelling up the container. Philo wrote this down in his book Pneumatica (using the Greek “pneuma” meaning “wind”), although he thought that some of the air in the container was being turned into fire and escaping through the glass. This experiment was repeated in the 17th century by chemist John Mayow, who also used a mouse instead of a candle to show that breathing also relied on this component of air, which Mayow named spiritus nitro-aerus (the spirit bit referring to how it allows for life).
However, this spiritus nitro-aerus idea was quelled pretty quickly by a new idea called the phlogiston theory. German alchemist J. J Becher posited this idea in the 17th century, which stated that flammable substances were made up of two components: phlogiston (an entity released when the stuff was burned) and the dephlogisticated component, which was believed to be the “true form” of the original substance. For example, wood was just a combination of phlogiston and ash, the latter being the “true form” of wood. It all stemmed from the idea that as things burned, they got lighter. In terms of a flame going out after spending some time in a closed vessel (which nowadays we know is when the oxygen is used up), the idea there was that so much phlogiston had been released into the air that it was saturated, and therefore this “phlogisticated air” could not take any more phlogiston on and the reaction halted.
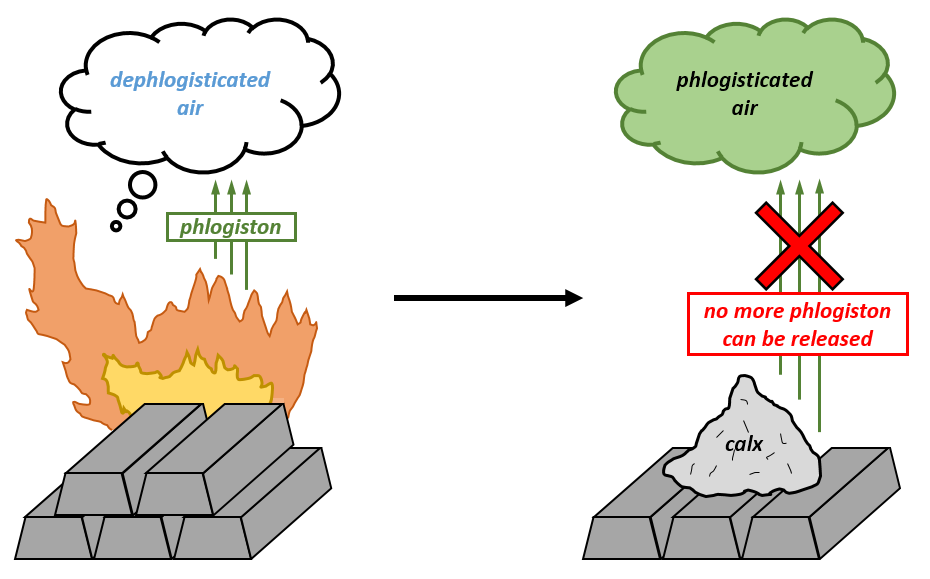
The very excellent if incorrect word phlogiston comes from the Greek word phlogistón meaning “burning up”. The phlogiston theory was further refined in the early 18th century by chemist Georg Ernst Stahl and one of his students Johan Pott, who even claimed that phlogiston was the basis of colours!
“But what about discovering oxygen?”, I hear you cry. Well that came later in the 18th century thanks to independent discoveries from Swede Carl Scheele in 1771, Englishman Joseph Priestley in 1774 and Antoine Lavoisier also in 1774. Scheel and Priestley generated oxygen through heating mercuric oxide, although Scheele called it “fire air” due to its combustibility and Priestley called it “dephlogisticated air” in line with the theory of the time. Lavoisier was the one to actively disprove the phlogiston theory and name oxygen as a new element. He figured this after heating tin and air in a sealed container and finding that the weight of the container not change, and that air rushed in when he opened the container as if air had been “used up” inside. This combined with his observation that the increase in weight of the heated tin was proportionate to the amount of air lost led him to conclude that something from the air was combining with the tin to make a new substance on its surface (now known to be tin oxide). He concluded that air had two parts: “vital air”, which was this combustible, breathable gas, and azote (“lifeless”) which was lifeless air. Vital air was changed by Lavoisier to oxygène (from Greek “oxy” meaning sharp in taste, like an acid, with “gen” meaning to produce) as further experimentation showed that this element was key in many acids. So yeah, oxygen literally means “produces acid”.



Battle of the chemists: Carl Scheele (left; from Store Norske Leksikon), Joseph Priestley (centre; from Wikimedia Commons) and Antoine Lavoisier (right, from Wikimedia Commons) all have claim to discovering oxygen.
So before we get onto the biological importance of oxygen, is there anything else we use oxygen for? Well apart from the chemical industry where oxygen-containing compounds like nitric acid, hydrogen peroxide, polyester, epoxyethene (antifreeze) and vinyl chloride, oxygen has an important use in the steel industry. Injecting oxygen into molten iron helps to remove impurities like sulphur and remove excess carbon by reacting with them to make sulphur and carbon dioxide respectively. This creates a purer, more effective steel.

So oxygen is an essential element to life, and this is typified by two molecules: water and diatomic oxygen. Water is two hydrogens bound to an oxygen atom (see all those internet experiments with “dihydrogen monoxide” for a fun demonstration of the demonisation of chemicals), and I don’t think I need to explain the importance of water, as the make up of our blood and cellular fluid. Diatomic oxygen- two oxygen atoms bonded together- is what is found in air. This is the oxygen that we breathe, passes from our lungs into our blood bound to haemoglobin in red blood cells, and passed around our bodies to where it can help us survive. But have you ever stopped and asked “why is oxygen so important?” If you have I will tell you.
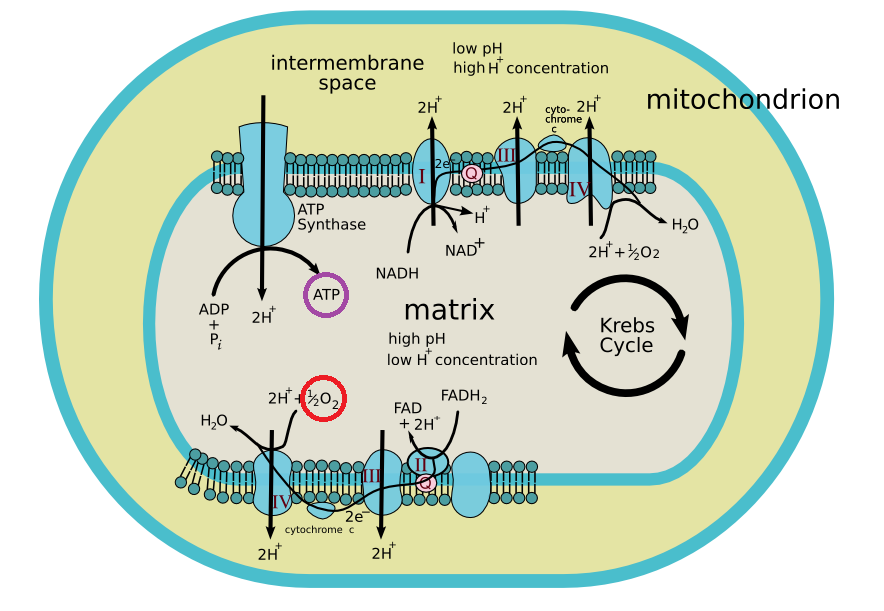
Many people believe respiration is the act of breathing air, and yes that is one definition. But in biochemistry, respiration is an incredibly important and complex reaction whereby biological molecules like fats and sugars are broken down, and with the help of oxygen provide us with energy to perform all the important tasks in our cells. The respiration reaction all takes place in our cells, but the final part takes place in a very special structure within the cell called the mitochondrion (plural mitochondria). The mitochondrion has two sets of membranes, one inside the other, creating two spaces: the inner matrix, and the intermembrane space. When those fats and sugars are broken down, they donate electrons to a set of proteins that span the membrane separating the intermembrane space from the matrix. These electrons then get shuttled down a chain of these proteins until they reach an enzyme that uses those electrons to combine hydrogen ions and oxygen to make water. This whole process releases energy, which is used to pump hydrogen ions out of the matrix and into the intermembrane space. Now with so much concentrated hydrogen ions in this intermembrane space, all they want to do is get back into the matrix, and so they go through a turbine protein called ATP synthase. This energy of flowing hydrogen ions (like a water wheel) drive the ATP synthase to create ATP, a molecule you may remember from our phosphorus post as one of the main energy sources for the cell. ATP can help catalyse reactions, replicate DNA and allow the cells to divide and grow. The take home message from my long attempt to explain respiration is that without oxygen to accept those electrons in the mitochondrion, we would not be able to generate energy in our cells!
Now if you’ve survived this very long article on oxygen thus far, I did promise the nastier side of oxygen, and it often occurs when this respiration reaction goes wrong. You see if an extra electron gets onto oxygen without the hydrogen ions joining it, you end up with reactive oxygen species. These highly reactive molecules can effectively react with and damage a bunch of things in your cells- proteins, membranes, even DNA! In fact, one of the hypotheses put forward about ageing is to do with reactive oxygenic species building up over time and attacking parts of your DNA that means they don’t replicate perfectly. This also occurs in photosynthesis, where energy absorbed from the sun is not used fast enough and so gets transferred to oxygen molecules, creating reactive oxygenic species that are dangerous to the chloroplast. So the fact that oxygen can accept electrons is both our source of energy and one of our greatest enemies!

And thus concludes my slightly long-winded post on oxygen. I hope you’ve enjoyed this ride, and thank you all for joining me through the first 100 elements. Only 18 left to go!

12 thoughts on “Day 100: Oxygen”